Trial Guides
Trial Guides’s mission is to make clinical trials more accessible to all patients by providing trusted, human navigation and transparent information about all available trial options.

“Human navigators make a huge difference for representation in trials.”
What are Trial Guides?
Trial Guides aims to fill a persistent gap – an ecosystem so festeringly clumsy and inefficient for patients that even senior clinical trial professionals are unable to easily find applicable trials when they are patients or caregivers.
Other industries have solved similar problems by establishing shared, trusted information layers. Air travelers can compare flights across providers. Home buyers can view current listings through multiple channels or from a human agent. These systems work because they provide accurate, up-to-date, standardized information upstream of any single agent or web platform.
Despite involving life-changing, often life-saving programs, clinical research lacks this foundational layer. As a result, trial discovery remains fragmented, unpleasant, and slow.
The Trial Guides initiative addresses this gap by creating a shared layer of Accessible Clinical Trial Information (A.C.T.I.) – clear, current, and standardized protocol-level data – supported by a trained and certified community of navigators and overseen by a cross-industry collective of pharma and clinical research leaders.
Patient Start
Patient screen fails at site OR
Insurer identifies possible match OR
HCP or patient advocate recommends the service
Authorization
Patient gives consent to connect to Trial Guides and share relevant data and contact information
Navigator Portal
Trial Guides identifies certified navigator in therapeutic area and links navigator to specific patient via the Navigator Portal
Navigation
Certified navigator reaches out to patient, uses A.C.T.I. data to answer questions and provides support to help patient enroll
Handoff
Navigator provides warm handoff to site, and patient enrolls in the study
TrialGuides
4 Essential Pillars
Trial Guides’s core thesis is that no single component (a better database, a better website, a better algorithm, or a single-company platform) can materially fix recruitment. Impact requires the combination of:
Actionable
DATA
Complete, machine readable, continuously updated and optimized
A.C.T.I. is a standardized trial data model optimized for recruitment and matching. It focuses first on capturing the Fundamental Five trial characteristics that materially improve screening and connections to sites.
Plain-language and machine-readable inclusion/exclusion criteria
Participant time commitment
Procedures and remote options
Participant support / reimbursement
Accurate site contact information (often missing or out of date today)
Certified NAVIGATORS
Educated and empathetic, trained and engaged with data
Objective, Trained Human Navigators
Certified Navigators provide empathy and education and use A.C.T.I. data to help patients connect to the trial that is best for them.
Unlocked THROUGHPUT
From existing screen failures and large covered entities
Sustainability Through Scale
Trial Guides is designed to unlock throughput from insurers and large covered entities, who could help connect many more patients to trials.
Ethical
FRAMEWORK
Nonprofit model with collaborative multi-sponsor buy-in
Governance Through a Cross-Industry Steering Committee
Neutral governance enables multi-sponsor trust and prevents dominance by any single company. The Steering Committee establishes standards, certification requirements, and transparency rules, ensuring Trial Guides serves the ecosystem and patients.
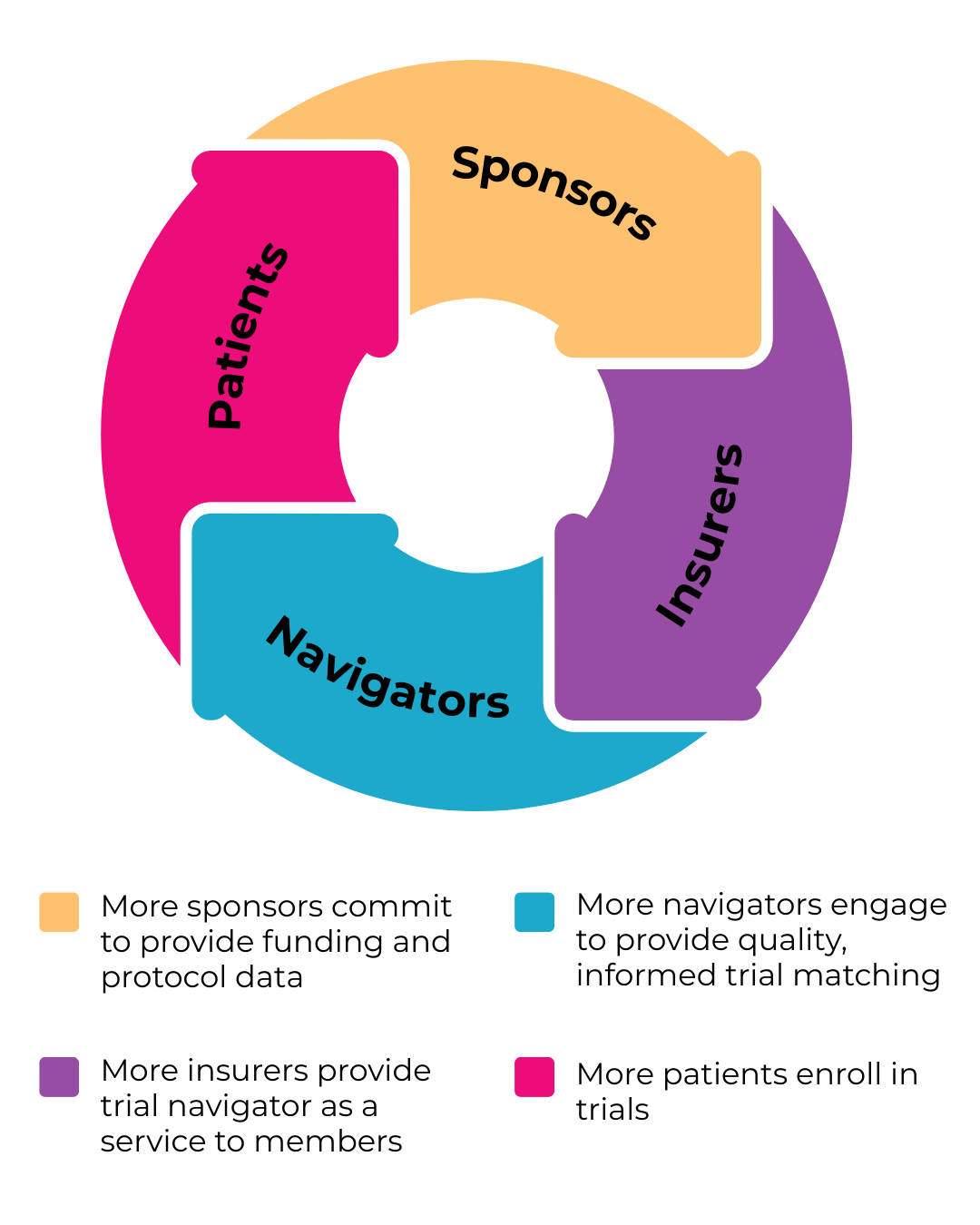
Stakeholders
Trial Guides brings together a diverse group of stakeholders united by the goal of improving patient access to clinical trials. CSL is taking a leadership role, and representatives from sponsors including BMS, Roche, Novartis, and Evinova have joined strategy sessions.
Other stakeholders include insurers, patient advocacy groups, nonprofits, and consortia. Trial Guides has the opportunity to benefit organizations across the industry:
-
Challenge: Unaware, or don’t know how to find options
Trial Guides Opportunity: Would benefit access to the whole field of options, with human support
-
Challenge: Face challenging trial designs and fluid regulatory environment
Trial Guides Opportunity: Could access new patient sources and make more of existing ones
-
Challenge: Struggle with engagement and pre-qualification
Trial Guides Opportunity: Could access pre-screened patients flowing from a curatable environment
-
Challenge: Remain “dabblers” in episodic, trial-by-trial recruitment
Trial Guides Opportunity: Would view Trial Guides as a broader, objective “insurance benefit” to their members
-
Challenge: Under-resourced despite critical advisory function for potential trial participants
Trial Guides Opportunity: Would have opportunities to both use AND develop their networks of disease-specific experts
-
Challenge: Increasingly encounter patient shortages with complex criteria expectations
Trial Guides Opportunity: Would benefit directly from greater volume of pre-validated candidates, especially at new locations
-
Challenge: Wait for pan-trial life cycle applications and standards to be agreed upon
Trial Guides Opportunity: Would see faster benefits early from critical sub-set of recruitment-specific standards
-
Challenge: Limit recruitment opportunities largely to existing sites
Trial Guides Opportunity: Would see volume increase for existing and new sites
For more information, contact Lucy Greenfield,
Lucy@SmithandJonesInnovation.com